Recently, the international journalStem Cell Research & TherapyA Phase 1 clinical study led by Zeinab Shirbaghaee's team exploring the feasibility and preliminary efficacy of treating patients with severe limb ischemia (CLI) using placenta-derived mesenchymal stem cells (MSCs) was published in the journal (Trial registration number: IRCT20210221050446N1).[1]The
A total of 9 male CLI patients were included in the study, including 2 diabetic foot and 7 thromboembolic vasculitis (TAO) patients, with a mean age of 55 years, and more than 90% of the participants' affected limbs had necrotic foot or long-term non-healing ulcers, which showed that the stem cell intervention presented a favorable therapeutic potential.

Severe limb ischemia (CLI), the most severe end stage of peripheral arterial disease (PAD), often manifests itself as rest pain, tissue loss, intractable ulcers, and even gangrene, which can ultimately lead to amputation.PAD is most often caused by atherosclerotic occlusive disease (ASO), and can be secondary to vasculitis such as thrombo-obscclusive vasculitis (TAO), and all of these etiologies significantly increase the risk of CLI .
stem cell therapySevere Limb Ischemia: Phase I Clinical Trial Report Safety and Feasibility | Patient Hope
The core objective of this trial is to assess the clinical feasibility and safety of placental MSCs transplantation for the treatment of patients with CLI, and to provide a basis for subsequent larger-scale clinical translation.
Methodology:Depending on the order of the trial, researchers randomized eligible patients into two MSCs groups using a 3 + 3 dose-escalation approach with 3-6 patients per MSCs dose cohort: 20 × 106 cells (low dose) and 60 × 106 cells (high dose).
MSCs were injected under spinal anesthesia (3 patients) and intravenous sedation (6 patients), and treatments were administered at 2 time points, 8 weeks apart. A suspension of MSCs was injected intramuscularly using a 30G needle to a depth of 1-1.5 cm and an area of 10 × 6 cm (30-40 sites, 0.5-1.0 ml MSCs per site).
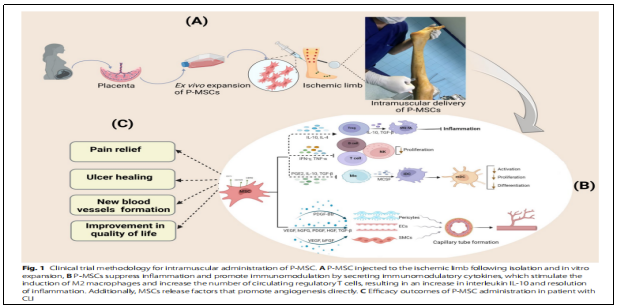
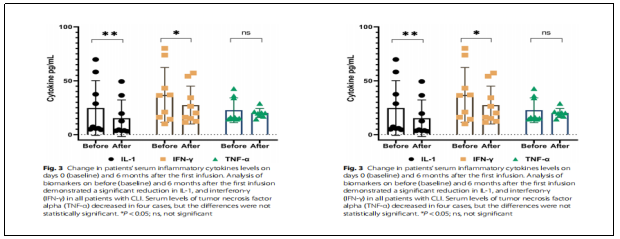
RESULTS: 1) Changes in immunological characteristics:IL-1 and IFN-γ serum levels were significantly reduced during the 6-month follow-up period after treatment (P ˂ 0.05, Fig. 3). No significant changes were observed when CD4, CD8, and CD25 markers were analyzed in patient blood samples using flow cytometry before and after treatment with MSCs (Fig. 4). This result suggests that the immune profile of CLI patients was not adversely affected by treatment with allogeneic MSCs.
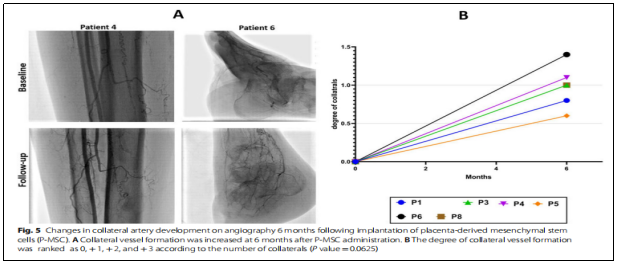
② Angiogenesis:Six months after cell infusion, the collateral angiogenesis score rose to 0.98±0.3 (Figure 5, +0 (no collateral circulation), +1 (mild) collateral circulation), + 2 (moderate collateral circulation), and + 3 (high collateral circulation)).
(iii) Pain relief:Resting pain was assessed using the VAS and showed significant improvement in all patients who completed the follow-up period (Figure 6A). The mean baseline VAS score was 8 (±0.75), which improved to 3.87 (±1.24) at 3 months and 1.125 (0.83) at 6 months.
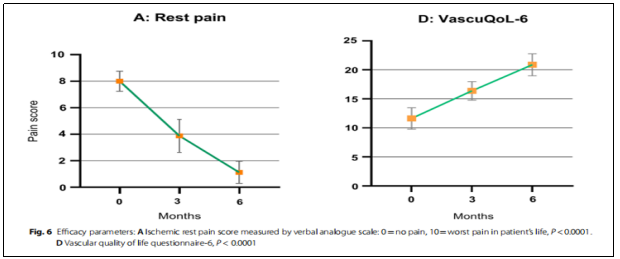
④VascuQoL-6 changes:There was a significant improvement in health-related quality of life after treatment (P˂0.0001, Fig. 6D), particularly in continuous walking and a significant reduction in the use of analgesics.
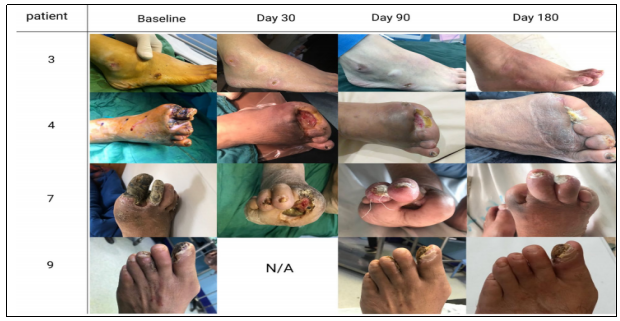
⑤ Ulcer changes:Throughout the 6-month follow-up period, one limb healed significantly; two limbs with ulcers and gangrene did not progress and remained completely dry; and one patient underwent a minor amputation prior to treatment versus five patients who underwent a post-treatment mini-amputation, which resulted in wound healing and a significant reduction in the total area of ulcers (see above).
(vi) Security:No patients experienced infection, bleeding, or other safety issues related to the cellular microorganisms after treatment; two patients experienced moderate diarrhea and two patients experienced mild itching after treatment, which improved on its own and the itching disappeared with antihistamine therapy.
Summary:The results of this phase I clinical study suggest that intramuscular MSCs are safe and tolerable and can significantly improve physical function and minimize inflammatory conditions in patients with CLI.
References:
[1]:Shirbaghaee, Z., Heidari Keshel, S., Rasouli, M. et al. Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with critical limb ischemia (CLI). Stem Cell Res Ther 14, 174 (2023). https://doi.org/10.1186/s13287-023-03390-9
Disclaimer: This article is intended only to disseminate scientific knowledge and share industry perspectives, and does not constitute any clinical diagnostic advice! The information published by Hangi Stem Cells is not a substitute for the professional advice of a physician or pharmacist. If you have any questions about copyright or other issues, please feel free to contact me.
郑重声明:本文版权归原作者所有,转载文章仅为传播更多信息之目的,如作者信息标记有错误,请第一时间联系我们修改或删除,多谢。

Leave a Reply