Spinal Cord Injury (SCI) results in localized neuronal death, as well as breaks in the axons that connect the brain to the spinal cord, both upstream and downstream. Since Ramon y Cajal's pioneering research more than a century ago, it has been recognized that severely injured axons in the adult central nervous system (CNS) are unable to spontaneously regenerate, which often results in permanent dysfunction, including motor, sensory, and autonomic functions below the plane of injury.
There is no approved clinical treatment for the acute protection or promotion of regeneration of neurons and axons following spinal cord injury, which is a huge unmet medical need.
Recent advances in the field of neural stem cell research have opened up new avenues for potential treatments for spinal cord injury (SCI). Typically, neural stem cell therapies offer three potential mechanisms for the treatment of spinal cord injury (SCI):
- (1) The damaged axonal pathway is "rewired" by transplanting neural stem cells (NSC) that differentiate into interneurons, which act as neuronal relays at the site of injury;
- (2) Myelin regeneration of remaining host axons around the injury site by transplantation of NSC-derived oligodendrocyte precursor cells (OPC);
- (3) Neuroprotection can be provided if NSC is injected early enough after injury.
Neural stem cell transplantation for spinal cord injury: analysis of repair mechanisms, clinical trial breakthroughs and translational prospects in 2025
On July 2, 2025, the Department of Neuroscience at the University of California, San Diego, USA, published an article in the internationally refereed journal journalTranslational NeuroscienceA review of the literature on neural stem cell therapy for spinal cord injury was published.[1]

The review study showed:Neural stem cells or neural progenitor cells (NPCs) transplanted into the site of severe spinal cord injury (SCI) survive, differentiate into neurons and glial cells, and extend a large number of axons over considerable distances in order to establish connections with host neurons below the injury site. In turn, host axons regenerate into NSC/NPC grafts and form synaptic connections with graft-derived neurons.
Thus, NSC/NPC graft-derived neurons can serve as neuronal relays to reestablish neurotransmission at the site of injury and improve functional outcomes even after severe SCI.
Isolation and characterization of neural stem cells (NSC)
Definition, origin and basic properties of neural stem cells:Neural stem cells (NSC) are early stem cells of the nervous system with the capacity for self-renewal and differentiation into neurons and glial cells (Fig. 1). They are mainly derived from embryonic stem cells (present in the inner cell mass of the blastocyst), and in response to specific transcription factors, a fraction of embryonic stem cells in the ectoderm differentiate into pluripotent neural stem cells that localize in the neural tube.NSCs, which are present for a species-specific period of time during development, rapidly expand and build the primitive nervous system.
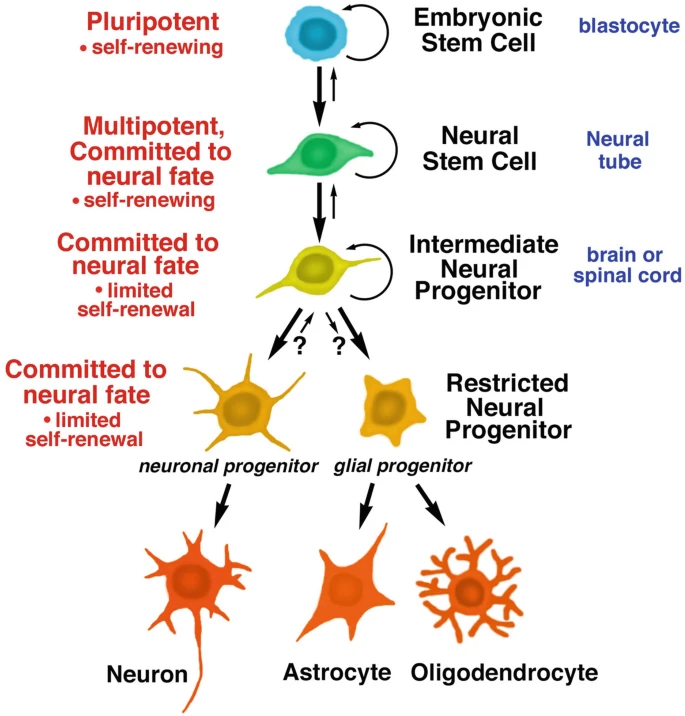
Figure 1: Developmental stages of embryonic and neural stem cells. All stem cells have the ability to divide and self-renew; daughter cells can differentiate into specific lineages, including neural stem cells, neural progenitor cells, which in turn differentiate into neurons and glial cells.
Differentiation pathways and specialized types of neural stem cells:After undergoing multiple divisions, NSCs enter an intermediate stage to become neural progenitors, which have a restricted capacity for self-renewal. These progenitors differentiate into two restricted precursors: neuron-restricted progenitors (which give rise only to neurons) and glial cell-restricted progenitors (which give rise only to astrocytes and oligodendrocytes).
Eventually, these precursor cells stop dividing and mature into specific cell types. In addition, a unique type of bipotent progenitor cell, the neuromesodermal progenitor (NMP), exists in the tail of the embryo and can differentiate into both neural lineages (e.g., spinal cord progenitor cells, neural crest cells) and mesodermal lineages.
The potential of neural stem cells in disease treatment:There are well-established protocols for inducing the differentiation of cultured NSC into functionally specific mature cell types for cell replacement therapy. For example, dopaminergic neurons can be induced to produce dopaminergic neurons for Parkinson's disease, striatal interneurons for Huntington's chorea, and cortical neurons for stroke.
NSC can also be induced to differentiate into oligodendrocytes for the treatment of disorders of myelin formation. Some NSC-based therapeutic strategies (e.g., dopaminergic neurons for Parkinson's disease) have entered clinical trials.
Neural stem cell therapeutic strategies for spinal cord injury:Two main NSC strategies have been explored in the treatment of spinal cord injury (SCI):
- One is the transplantation of pluripotent NSCs, which are expected to differentiate and replace neurons, astrocytes, and oligodendrocytes at the site of injury, forming new neural connections;
- The second is the transplantation of oligodendrocyte progenitor cells that have been directionally differentiated, aiming to regenerate myelin sheaths for residual host axons to restore partial function.
A third potential mechanism is the secretion of neuroprotective factors (e.g., growth factors) by the grafted cells, which play a protective role early in the injury.
Where do neural stem cells come from to treat spinal cord injuries?
Diverse sources of neural stem cells:Early studies primarily used NSC or tissue from the developing spinal cord or brain. Today, there is a wider range of alternative sources of NSC and its derivatives:
- (1) Directly differentiated from embryonic stem cells;
- (2) From induced pluripotent stem cells (iPSC, obtained by reprogramming of mature somatic cells);
- (3) Direct reprogramming of somatic cells into NSCs using specific combinations of transcription factors.
These different sources provide additional options for research and treatment, and these cell sources are described in more detail below.

Table 1: Sources of neural stem cells and neural progenitor cells
01. Neural stem cells isolated directly from the developing spinal cord
Neural stem cells (NSC) and progenitor cells undergoing directed differentiation to neurons or glial cells can be isolated directly from the developing spinal cord on days 11 to 18 of rodent embryonic development or weeks 8 to 10 of human embryonic development.
The key advantage of such cells is that they have a spinal cord-specific cell fate, i.e., they tend to differentiate into cell types suitable for the spinal cord environment (e.g., spinal cord excitatory interneurons), which is essential for the re-establishment of functional synaptic relays at the site of spinal cord injury (SCI). Based on this advantage, such NSCs derived from the developing spinal cord have been used in clinical trials for the treatment of amyotrophic lateral sclerosis (ALS) and spinal cord injury.
02. Transformation of embryonic stem cells (ESC) into neural stem cells
Embryonic stem cells (ESC) (derived from cell clusters within blastocysts) have the potential to differentiate into almost all cell types, including neural stem cells. While human and mouse ESC lines are abundant, ESC lines from rats, a commonly used model for SCI, are relatively scarce.
The use of ESC allows the generation of neural stem cells and their derivatives for therapeutic purposes: early studies have generated oligodendrocyte progenitor cells (OPC) for axonal myelin regeneration, and now it is also possible to generate NSC for neuronal and glial cell replacement therapy. In particular, recent techniques have enabled the stable generation from human ESCs and the long-term maintenance of NSCs with spinal cord properties in vitro.
03. Transformation of induced pluripotent stem cells (iPSC) into neural stem cells
In 2006, Shinya Yamanaka's team was the first to convert adult cells (e.g., fibroblasts) into induced pluripotent stem cells (iPSCs) by reprogramming them with a specific transcription factor. The subsequent demonstration that iPSC can also differentiate into neural stem cells holds great promise for the repair of neurological damage using neurons and glial cells derived from a patient's own cells.
The strategy of inducing iPSCs into NSCs for autologous transplantation has now entered the clinical trial stage for spinal cord injury and amyotrophic lateral sclerosis (ALS) in Japan.
04. Direct differentiation of somatic cells into neural stem cells
Recently, technologies have been developed that can directly drive the differentiation of mature (or even "post mitotic") somatic cell types into other cell types.
Core techniques and processes for direct reprogramming:Somatic cells (e.g., fibroblasts, hematopoietic cells) can be transformed into neural stem cells (NSCs) or even mature neurons without having to be first reversed to a pluripotent stem cell state by direct reprogramming with specific combinations of transcription factors (e.g., Sox2, Klf4, c-Myc, Brn2, Brn4, E47/Tcf3, FoxG1). The process typically starts with easily accessible somatic cells (e.g., skin biopsy-derived fibroblasts), which are induced to express these key transcription factors in an in vitro culture system. By precisely optimizing the cell culture substrate, adding the necessary growth factors and small molecule compounds, and culturing the cells in vitro for a period of time (typically 1 to 2 months), a population of cells with neural stem cell properties is ultimately obtained.
Advantages, Applications and Prospects:Neural stem cells (sometimes referred to as induced neural stem cells, iNSC) generated by this direct reprogramming technique are a potential source of cellular therapies for diseases such as spinal cord injuries, along with embryonic stem cell (ESC) or induced pluripotent stem cell (iPSC)-derived NSCs, as well as neural progenitor cells derived from the developing spinal cord. Significant advantages include avoidance of the pluripotent stem cell stage, potentially shorter time and reduced risk of tumorigenesis, and autologous transplantation potential, especially when using the patient's own cells.
Although these different sources of NSCs still need to be thoroughly studied in terms of characterization, epigenetic imprinting, safety (e.g., chromosomal stability), and eventual differentiation potential, this technique provides a new and important avenue for obtaining patient-specific neural stem cells for repair (e.g., differentiation into oligodendrocyte precursors for myelin regeneration), and relevant clinical applications are being explored.
In vivo study identifies three major mechanisms of action of neural stem cells for spinal cord injuries
1. Neuroprotective mechanisms:Neural stem cell (NSC) transplantation can exert neuroprotective effects by secreting neurotrophic factors (e.g., VEGF, FGF-2) and modulating the immune microenvironment. Studies have shown that transplanted NSC can regulate immune cytokines (e.g., IL-1α, IL-6, IL-10) at the early stage of injury (e.g., within 10 days after injury), reduce inflammation and cell death, and promote angiogenesis, thus protecting the residual spinal cord tissues and reducing the extent of injury. This protective effect creates a more favorable microenvironment for subsequent repair.
2. axonal myelin regeneration strategies:For functional recovery of residual axons after spinal cord injury, research has focused on myelin regeneration by transplanting oligodendrocyte precursor cells (OPCs). Embryonic stem cell (ESC) or induced pluripotent stem cell (iPSC)-differentiated OPCs implanted around the periphery of the injury zone (rather than in the injury cavity) can encapsulate host axons to form myelin sheaths, reduce cavities, and improve nerve conduction.
For example, Tsuji's team demonstrated in mouse experiments that iPSC-derived NSCs promote myelin regeneration and axon growth, and has initiated the world's first related clinical trial (jRCTa031190228) by transplanting human iPSC-NSCs in patients within 24 days of injury.
3. Functional nerve relay reconstruction:The core potential of NSC is to differentiate into neurons that form "neural relays" across the injured area. Transplanted NSC can differentiate into excitatory interneurons that replace lost neurons and act as "bridges" to reconnect broken upstream and downstream neural pathways.
Meanwhile, its differentiated glial cells (e.g. astrocytes) can support host axon regeneration. This strategy of combining neuronal replacement with circuit reconstruction, aimed at restoring motor sensory conduction function, is a key direction of current research.
Clinical trial of neural stem cell transplantation for the treatment of spinal cord injury
Clinical trials of NSC/NPC began in the late 1990s with the transplantation of human fetal spinal cord tissue into SCI patients with spinal cord cavernous disease, with the primary goal of preventing further expansion of the cysts. The trial was inconclusive and no functional recovery was reported. Since then, several phase I/II clinical trials have been conducted using neural stem cells from different sources.
The primary goal of all these studies was to remyelinate axons retained after the initial injury and possibly protect the remaining spinal cord tissue from secondary degeneration. These experiments were based on three major cellular sources.
1. Clinical trials of embryonic stem cell-derived oligodendrocyte progenitor cells (OPCs)
In 2011, oligodendrocyte progenitor cells (OPC) derived from human embryonic stem cells (ESC) were the first ESC-derived cell product to be tested in human trials for the treatment of spinal cord injury.
In 2012, Geron sponsored a first-in-human clinical trial evaluating human embryonic stem cell therapy. The trial was an initial Phase I study of five patients with SCI in the thoracic spine and demonstrated its safety.
2022 in a phase 1/2a dose-escalation study of oligodendrocyte progenitor cells for the treatment of patients with subacute cervical spinal cord injury (NCT02302157, 25 patients with cervical spine injury).

The results clearly demonstrate its safety and also show that almost all patients regain nerve function in one segment and one-third of patients regain nerve function in at least two segments on one side of the body. This study provides valuable information that can be used to evaluate the next steps in stem cell therapy for subacute SCI.
2. Research and termination of fetal brain neural stem cells (HuCNS-SC®)
Stem Cells Inc. conducted two clinical trials based on fetal brain neural stem cells (NSCs): the first targeted 12 cases with thoracic spine injuries (NCT01321333) and the second was planned to enroll 31 patients with chronic cervical spine injuries (NCT02163876). The studies showed that the transplanted cells were generally safe, but the functional improvement did not meet the pre-determined thresholds, leading to early termination of the program and the company's eventual discontinuation of the spinal cord injury research program. This result highlights the limitations of fetal-derived stem cells in promoting significant functional recovery.
3. Fetal spinal cord neural stem cells (NSI-566) and other ongoing trials
In 2018, a first-in-human Phase I study of neural stem cell transplantation for chronic spinal cord injury was conducted to test the feasibility and safety of human spinal cord-derived neural stem cell (NSI-566) transplantation for chronic spinal cord injury (SCI).

First Human Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury
In this clinical trial, four patients with T2-T12 spinal cord injuries (SCI) were treated with removal of internal spinal fixation, laminectomy, and durotomy followed by six midline bilateral stereotactic injections of NSI-566 cells. The procedure was well tolerated by all subjects.There have been no serious adverse events to date (18-27 months post-transplant).
In two subjects, theOne to two levels of neurologic improvement were detected using the ISNCSCI motor and sensory scores. Our findings support the safety of NSI-566 transplantation into spinal cord injury sites, and early indications of potential efficacy in three of these subjects warrant further exploration of NSI-566 cells in dose-escalation studies.
- What are the long-term clinical outcomes of neural stem cell transplantation for spinal cord injury? How safe is it?
In January 2025, scientists from the University of California and other research institutions, after five years of clinical follow-up, published a study in the journal Cell Reports Medicine entitled "Long-term clinical and safety outcomes from a single-site phase 1 Long-term clinical and safety outcomes from a single-site phase 1 study of neural stem cell transplantation for chronic thoracic spinal cord injury" in the journal Cell Reports Medicine, the researchers achieved some encouraging results:
Long-Term Clinical and Safety Outcomes of a Single-Center Phase I Clinical Study of Neural Stem Cell Transplantation for Chronic Thoracic Spinal Cord Injury
Transplantation safety was verified:All four patients tolerated the procedure well with no serious adverse events (SAEs). Although one of the patients passed away due to postoperative complications of infection, overall, neural stem cell transplantation demonstrated a high safety profile in both the short and long term.
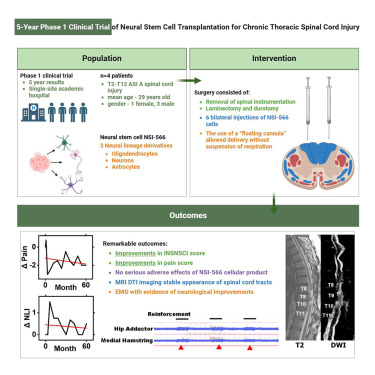
Neuromotor and sensory functions were improved:The study reported that all four subjects tolerated the stem cell transplantation procedure well, and two of them obtained evidence of durable EMG-quantified neurologic improvement and improved neuromotor and sensory scores 5 years after transplantation. The results of the other two, a Korean study using ESC-derived PSA-NCAM-positive neural precursor cells (NCT04812431) and a Japanese study transplanting iPSC-derived neural stem cells into subacute patients, have not yet been published.
4. Common challenges and future directions
All of these trials used a strategy of injecting cells around the periphery of the injury area (or partially into the injury cavity) and none of them combined growth factors to support graft cell survival. The study suggests that the lack of growth factors may contribute to the difficulty of long-term graft survival in the core of the injury. Although all trials were unable to validate efficacy due to small sample sizes, they consistently confirmed the basic safety of stem/progenitor cell transplantation. Future work is needed to optimize cell delivery strategies, incorporate neurotrophic factors or biomaterials, and validate functional recovery in larger trials.
Future perspectives of neural stem cell therapy for spinal cord injury
Axonal growth of transplanted neural stem cells (NSC) and their reconnection with host neurons holds promise for the treatment of spinal cord injury (SCI). Our approach aims to form reparative neural relays at the site of injury. We believe that our approach is mature enough to warrant clinical trials.
Core Strategies and Clinical Translational Potential:The central goal of neural stem cell (NSC) transplantation is to promote newborn axon growth and reconnection with host neurons by constructing functional neural relays at the site of injury. This strategy has evolved from preclinical studies to a mature stage that is ready for human clinical trials.
Future work will focus on optimizing transplantation protocols, such as combining NSCs with bioengineered scaffolds to guide axon directed extension and form more precise neural circuit connections; at the same time, by delivering neurotrophic factors or axon guidance molecules (e.g., Netrin, Slit, etc.), graft axons can be specifically guided to target the host's motor control regions to enhance the precision and functional efficiency of neural relay.
Efficiency-enhancing technologies and synergistic challenges:Further efficiency strategies include:
(1) Enrichment of excitatory neurons (e.g., spinal V2a interneurons) in grafts to enhance downstream motor signaling;
② Activates the regenerative potential of adult axons and promotes their integration with transplanted neurons by inhibiting the host PTEN gene, among other pathways;
(iii) Combining customized rehabilitation training with epidural electrical stimulation to strengthen the plasticity and functional adaptation of the new circuit.
However, it is important to be aware of the risk that axonal "overgrowth" may lead to abnormal neural connections. Currently, the field has broken through the bottleneck of "whether regeneration is possible" and is now moving towards the new stage of "how to precisely guide regeneration" - optimizing the direction, safety and functional integration of nerve growth is the core challenge for future clinical trials. Optimizing the direction, safety and functional integration of nerve growth are the core challenges for future clinical trials.
Reference:[1]:Lu, P., Sinopoulou, E., Rosenzweig, E.S., Blesch, A., Tuszynski, M.H. (2025). Neural Stem Cells for Spinal Cord Injury. In: Tuszynski, M.H. (eds) Translational Neuroscience. Springer, Cham. https://doi.org/10.1007/978-3-031 -89307-0_14
Disclaimer: This article is intended only to disseminate scientific knowledge and share industry perspectives, and does not constitute any clinical diagnostic advice! The information published by Hangi Stem Cells is not a substitute for the professional advice of a physician or pharmacist. If you have any questions about copyright or other issues, please feel free to contact me
郑重声明:本文版权归原作者所有,转载文章仅为传播更多信息之目的,如作者信息标记有错误,请第一时间联系我们修改或删除,多谢。

Leave a Reply