Spinal cord injury (SCI) is a devastating trauma to the central nervous system, usually caused by an external mechanical force that damages the structures of the spinal cord, resulting in motor, sensory and autonomic dysfunction below the level of injury. With more than 15 million patients worldwide and an estimated 3 million patients in China, and about 100,000 new cases every year, SCI not only causes lifelong disability, but also brings tremendous psychological burden and socio-economic pressure.
Traditional treatments, such as surgical decompression, medications and rehabilitation, can stabilize the spine to a certain extent, reduce secondary injuries and improve some functions.However, none of them can achieve true regeneration of neural structures and complete functional recoveryThe presence of a blood-spinal cord barrier limits drug delivery. The presence of a blood-spinal cord barrier limits drug delivery, and the inherent low regenerative capacity of the CNS makes repair even more difficult.
In the face of this dilemma, stem cell therapy has become the most promising therapeutic direction in the field of regenerative medicine by virtue of its unique self-renewal, multidirectional differentiation, and secretion regulation capabilities. Mesenchymal stem cell therapy for spinal cord injury and neural stem cell therapy for spinal cord injury are the two mainstream strategies in the current clinical research of spinal cord injury, and each of them promotes nerve repair through unique mechanisms, which brings new hope for the functional recovery of spinal cord injury patients. So which of these two therapies is more suitable for specific types of spinal cord injury patients? How should we choose scientifically? In this article, we will analyze the mechanism of action, clinical evidence and application scenarios of these two therapies to help readers form a rational judgment of the two therapies and provide reference for actual treatment decisions.
Are mesenchymal stem cells more effective in treating spinal cord injuries? Or neural stem cells?
I. Overview of stem cell types
1.1 Mesenchymal stem cells
source (of information etc): MSCs are mesodermally derived pluripotent adult stem cells obtained from a wide range of sources, including bone marrow, adipose tissue, umbilical cord and placenta. Among them, umbilical cord-derived MSCs (UC-MSCs) are favored in clinical applications because of their high proliferative capacity, low immunogenicity, and lack of ethical controversy.
Basic Characteristics: The core strength of MSCs is not their direct differentiation into neuronal cells, but their strong immunomodulatory and paracrine functions . They can secrete a variety of neurotrophic factors (e.g., BDNF, GDNF), anti-inflammatory factors (e.g., IL-10), and pro-angiogenic factors (e.g., VEGF), which can improve the damaged microenvironment, inhibit apoptosis, and promote endogenous repair. In addition, MSCs are easy to amplify in vitro and can be autotransplanted, avoiding the problem of immune rejection
1.2 Neural stem cells
source (of information etc)NSCs are a class of stem cells found in the nervous system and can be obtained directly from fetal brain or spinal cord tissue. However, with the development of reprogramming technology, the targeted differentiation of NSCs by induced pluripotent stem cells (iPSCs) has become a mainstream trend, which not only solves the ethical issues, but also offers the possibility of "off-the-shelf" cell therapy.
Basic Characteristics: The core ability of NSCs is their multidirectional differentiation potential, which allows them to directly differentiate into the three major types of cells that make up the nervous system: neurons, astrocytes, and oligodendrocytes. Therefore, NSCs transplantation aims to directly replace damaged nerve cells, rebuild nerve circuits, and promote myelin regeneration to restore signaling.
| characterization | Mesenchymal Stem Cells (MSC) | Neural Stem Cells (NSC) |
|---|---|---|
| Primary sources | Bone marrow, fat, umbilical cord | Fetal neural tissue, iPSC differentiation |
| differentiation potential | Limited neural differentiation, predominantly differentiated into supporting cells | Can differentiate into neurons, astrocytes, oligodendrocytes |
| Main mechanisms | Immunomodulation, secretion of neurotrophic factors, promotion of angiogenesis | Replacement of damaged neurons, reconstruction of neural networks, formation of myelin sheaths |
| dominance | Low immunogenicity, easy to obtain, good safety profile | Strong neural directed differentiation and high integration efficiency |
| limitations | Limited capacity for neural differentiation | Restricted sources, ethical controversies |
II. Comparison of mechanisms of action
2.1 Main Mechanisms of Action of MSCs
The main mechanism by which MSCs treat spinal cord injury is not limited to differentiation and replacement, but is achieved through multiple paracrine effects:
anti-inflammatory effect: Regulates the conversion of microglia from pro-inflammatory M1-type to anti-inflammatory M2-type, reduces the levels of inflammatory factors such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and attenuates secondary injury;
Growth factor secretion: Produces a variety of neurotrophic factors, such as BDNF, GDNF and NGF, which promote neuronal survival and axonal regeneration;
Promotes angiogenesis: Improvement of local blood supply to the injury and nutritional support for nerve repair through secretion of factors such as VEGF and Angiopoietin-1;
Reduce apoptosis: Inhibits the expression of apoptosis-related proteins such as Caspase-3 and protects damaged but not yet dead neuronal cells.
2.2 Main mechanisms of action of neural stem cells
NSCs, on the other hand, function mainly through direct substitution and structural reconstruction:
Replacement of damaged neurons: differentiate into functional neurons that replace neuronal cells that have died due to injury and are directly involved in neural signaling. Studies have shown that transplanted NSCs can differentiate into a variety of functional cells such as motor neurons (accounting for 161 TP3T), V2 interneurons (accounting for 111 TP3T) and dI4 interneurons (accounting for 29.41 TP3T);
Facilitating neural network reconstruction: Extended nerve fiber and host neuron formationsynaptic connectionThe neural signaling pathway was re-established. Immunofluorescence staining confirmed the co-localization of human-specific synaptic protein (hSyn) expressed by the transplanted cells with host neuromarkers (MAP2), suggesting that "signaling interoperability" was truly achieved;
myelin sheath formation: differentiates into oligodendrocytes that wrap around damaged axons to form myelin sheaths and restore nerve conduction velocity;
Regulation of the microenvironment: A recent study found that NSCs also have the ability to regulate the local microenvironment of injury, inhibiting glial scarring and "opening the way" for axonal regeneration.
III. Comparison of evidence from clinical and experimental research data
3.1 Clinical data on mesenchymal stem cell therapy for patients with spinal cord injury
On March 6, 2025, the media conference of "Stem Cell Clinical Research - Spinal Cord Injury Patient Recruitment for the Record of the Two National Commissions" was held, and Xi'an Hospital formally launched theNorthwest's First CNS Injury Stem Cell Program. The project aims to achieve this throughMesenchymal stem cell transplantation for spinal cord injuryIt marks an important clinical breakthrough from "irreversible" damage to "functional reconstruction".[1]

At the conference, the dean introduced in detail the background and process of the project "Mesenchymal Stem Cell Transplantation for Spinal Cord Injury". He pointed out thatAfter trying many methods such as surgery and medication, the condition never progressed over the years and stayed at the same level of efficacy. Traditional treatments can only clean up the damaged area through surgical decompression, but cannot achieve regenerative repair of the nerve signaling pathway.The
Based on this, Dr. He led the team to start the research of stem cell therapy, hoping to rebuild the "communication network" of the damaged nervous system through precise stem cell transplantation technology, and provide new treatment pathways for patients.
Director Wei Zhang shared the initial results of the program at the meeting: of the six spinal cord injury patients who participated in the clinical study, theFive patients showed improvement in neurological function and four patients showed significant improvement in motor function, with two patients showing particularly impressive recovery. A patient with a complete injury has been able to stand briefly with assistance after treatment, demonstrating the feasibility of cell transplantation for spinal cord injury and providing a strong case for further clinical trials.
A 22-year-old spinal cord injury patient in Shanxi was confined to a wheelchair after a fall from a height, and his lower limbs continued to thrash and twitch, making it difficult for him to even sit in bed.After the stem cell transplant, the muscles of the lower limbs are gradually getting stronger and he is now able to roll his back and kneel by himselfThe
Another patient with a spinal cord injury, who normally walks only with the aid of crutches, one month after cell transplantation, theHe's been able to throw away his crutches and walk easily.The

3.2 Clinical data on neural stem cell transplantation for the treatment of patients with spinal cord injuries
On December 17, 2024, researchers at the University of California, San Diego School of Medicine published an article in the trade journal Cellular Medicine Reports on the long-term clinical and safety results of a single-center, Phase I clinical study of neural stem cell transplantation for chronic thoracic spine injury.[2]
This published Phase I clinical study was designed to evaluateSafety and preliminary efficacy of neural stem cell transplantation for chronic thoracic spinal cord injury. Four patients with complete ASIA-A grade thoracic SCI were selected as subjects for the study, all of whom had been injured for more than one year and had not shown significant improvement after any other form of effective treatment.

Each subject received an injection containing 2 × 10 at each injection site.5Individual neural stem cells are injected bilaterally into the remaining tissue around the injury site and into the medial white matter area approximately one segment below through a customized stereotactic device, with the entire process guided by intraoperative fluoroscopic imaging.
Clinical Outcomes:
1. Overview of neurological responses:By ISNCSCI follow-up, 2 subjects (001, 010) showed significant neurologic improvement after transplantation: 001 had risen by two segments at 2 years post-transplantation, and then fell back to a 1-segment rise at 5 years; 010 maintained a steady neurologic improvement throughout the 5-year follow-up period.
2. Immunosuppression and immune response:All four subjects discontinued tacrolimus and mycophenolate mofetil at 12 weeks post-transplantation; although positive anti-HLA antibodies were detected in one case (010) at 6 months, they were not directed against donor-specific antibodies, and the team concluded that this was not an immune rejection directly caused by the NSC transplant.
3. Pain and quality of life:Regarding pain, 001 and 006 reported a decrease in overall pain, 008 had increased pain and a significant decrease in self-care in life (SCIM) due to a serious adverse event at 30 months from a sacral ulcer, 010 had less change in pain and SCIM, and overall no significant improvement in SCIM was seen in 3 patients.
4. Imaging evaluation:Postoperative MRI showed varying degrees of focal spinal cord tenderness in all patients, but no new concurrent imaging signs (e.g., edema, enhancement, or effusion) were seen; DTI suggested a stable appearance of the spinal cord bundles, with no extensive remodeling or improvement of the fiber bundles.
5. Neurophysiologic response:Electrophysiologic examinations showed new EMG activity with responses to reinforcing maneuvers in several patients in the months to years after transplantation (e.g., 001, 006, and 010 showed new-onset EMG signals and enhanced muscle responses in different segments), suggesting signs of functional neuromuscular recovery in some of the segments.
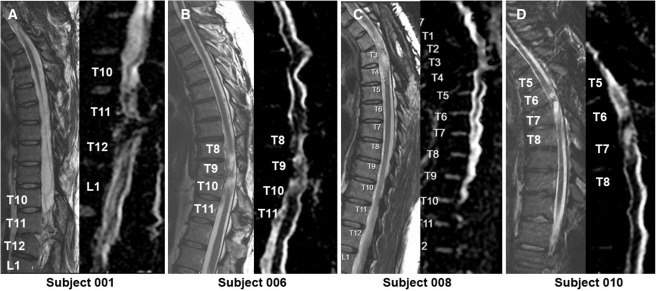
The findings suggest that neural stem cell transplantation is a feasible and relatively safe method to promote neurologic recovery in patients with chronic thoracic SCI to some extent.
On July 2, 2025, the Department of Neuroscience at the University of California, San Diego published a literature review on "Neural Stem Cell Therapy for Spinal Cord Injury" in Translational Neuroscience, a leading international journal.[3]

The review study showed:Neural stem cells or neural progenitor cells (NPCs) transplanted into the site of severe spinal cord injury (SCI) survive, differentiate into neurons and glial cells, and extend a large number of axons over considerable distances in order to establish connections with host neurons below the injury site. In turn, host axons regenerate into NSC/NPC grafts and form synaptic connections with graft-derived neurons.
Thus, NSC/NPC graft-derived neurons can serve as neuronal relays to reestablish neurotransmission at the site of injury and improve functional outcomes even after severe SCI.
3.3 Direct comparison and synthesis
There are very few head-to-head clinical studies comparing the efficacy of MSCs and NSCs. However, from the available data:
safetyThe clinical safety record of MSCs is more extensive and mature, and the application is easier (intravenous/intrathecal administration is possible).Transplantation of NSCs usually requires precise intra-lesional injection, which is more invasive, and its long-term safety (e.g. tumorigenicity) still needs more data to be evaluated. The transplantation of NSCs usually requires precise intra-lesional injection, which is more invasive, and its long-term safety (e.g., tumorigenicity) needs to be evaluated with more data.
validity: MSCs are outstanding in regulating the immune microenvironment and protecting the residual neural tissue, especially in the acute and subacute phases. While NSCs have more potential in structural reconstruction (cell replacement), they can directly replace damaged neurons, rebuild neural networks, break through the obstacles of glial scarring, and are more beneficial to patients in chronic phase is more favorable.
Aspects of functional recovery effects: MSCs primarily improve ASIA scores and somatosensory evoked potentials but have a limited impact on certain neurologic functions, whereas NSCs lead to more comprehensive improvements in sensory and motor functions, including fine motor control and gait coordination.
Summary and outlook
Taken together, the available evidence suggests that MSCs and NSCs each have their own advantages in the treatment of spinal cord injuries, and their applicability depends on the stage of injury, type of injury, and therapeutic goals.MSCs excel in modulating inflammation and improving the microenvironment in the acute phase; NSCs have greater potential for neural replacement and circuit reconstruction in the chronic phase. The future therapeutic trend is to develop combined strategies and incorporate new technologies such as genetic engineering and 3D scaffolds to ultimately achieve meaningful functional recovery for patients.
郑重声明:本文版权归原作者所有,转载文章仅为传播更多信息之目的,如作者信息标记有错误,请第一时间联系我们修改或删除,多谢。

Leave a Reply